1b Feel the pressure

Background
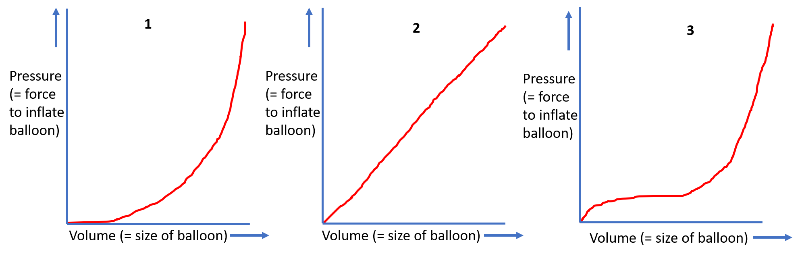
Rubber consists of long chain molecules that form a network. This network with its entangled molecules is the secret behind the ability to stretch so much. Upon stretching the chain molecules are aligned until they are all straight resulting in an increase in the resistance to stretch. When you inflate a rubber balloon the volume (= size) of the balloon and the pressure (= force you need to inflate the balloon) have a dependency on another. Can you feel how much a rubber ballloon resist the inflation with increasing size?
What do you need?
rubber balloon
What do you have to do?
1 Take a balloon and start inflating it. [br][br]2 Try to feel how hard and easy it feels to inflate the balloon depending on the size.[br][br]Is it harder to inflate the completely empty balloon, is there a point where it feels easy, can you inflate the ballloon so much that it pops?[br][br]Select the correct pressure versus volume curve (=force you need to inflate the balloon versus size) from the graphs below and type the number. [br][br][br][br]